Self-driving, or autonomous cars, are revolutionizing the way the public looks at travel and car ownership. These vehicles turn active drivers into passive passengers, allowing motorists to rely on the car’s advanced computerized system to navigate the roads and avoid collisions. However, these cars may result in a serious Texas car accident, as the new technology is still being refined.
Self-driving, or autonomous cars, are revolutionizing the way the public looks at travel and car ownership. These vehicles turn active drivers into passive passengers, allowing motorists to rely on the car’s advanced computerized system to navigate the roads and avoid collisions. However, these cars may result in a serious Texas car accident, as the new technology is still being refined.
Autonomous vehicles rely on complex computer systems, sensors, actuators, and various algorithms to operate on the roads without an active driver. In theory, these cars provide a glimpse into a more environmentally friendly and safer future for road users. However, as it is, these features often present more dangers than benefits.
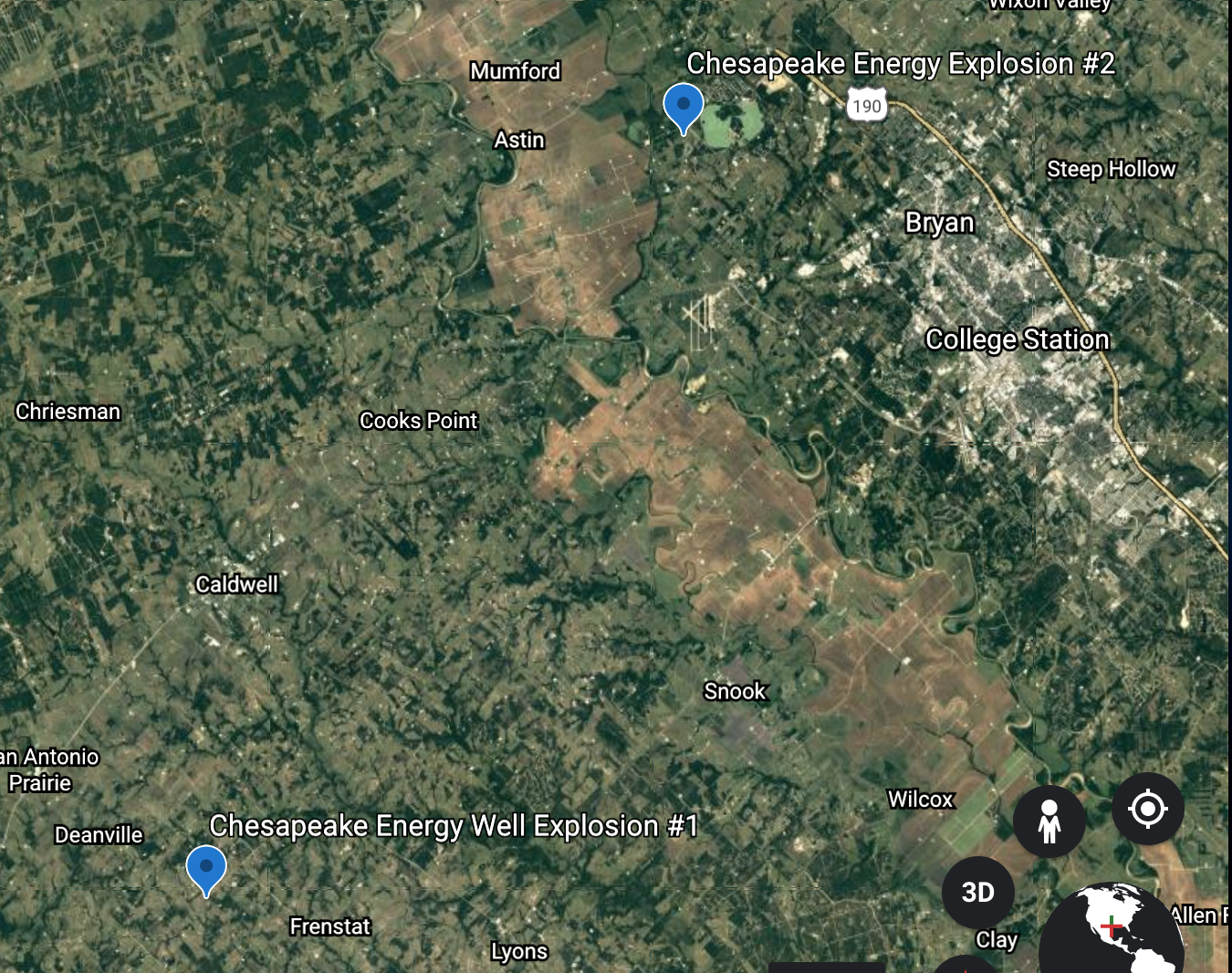
For example, recently, a national news report described a fatal Tesla crash involving a driverless vehicle. According to reports, the vehicle did not have a driver and was operating on high or full automation mode. As such, one of the occupants was in the front passenger seat, and the other occupants were in the back seat. The car was speeding along a dangerous curve when it slammed into a tree. Emergency responders used over 30,000 gallons of water to put out the massive fire that the collision sparked. Tesla did not respond to this incident but previously stated that their vehicles are intended to be used with an attentive driver who has their hands on the steering wheel. However, safety officials argue that the company does not do enough to deter drivers from depending too much on the vehicle’s features.
 Texas Injury Lawyers Blog
Texas Injury Lawyers Blog


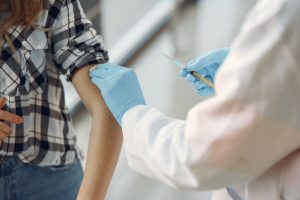 Vaccines are one of the most effective ways to prevent the spread of infectious diseases throughout the world. The overwhelming majority of people who receive vaccines do not experience serious problems, and the benefits greatly outweigh the threat of risk. However, similar to the risks associated with natural supplements and other pharmaceuticals, some vaccine recipients may suffer adverse reactions. Depending on the nature and circumstance of the injury, victims may file a Texas medical malpractice or pharmaceutical error lawsuit. Further, the National Vaccine Injury Compensation Program (VICP) provides compensation to individuals who have suffered injuries from certain vaccines.
Vaccines are one of the most effective ways to prevent the spread of infectious diseases throughout the world. The overwhelming majority of people who receive vaccines do not experience serious problems, and the benefits greatly outweigh the threat of risk. However, similar to the risks associated with natural supplements and other pharmaceuticals, some vaccine recipients may suffer adverse reactions. Depending on the nature and circumstance of the injury, victims may file a Texas medical malpractice or pharmaceutical error lawsuit. Further, the National Vaccine Injury Compensation Program (VICP) provides compensation to individuals who have suffered injuries from certain vaccines. During the course of the COVID-19 global pandemic, tens of millions of people across the country experienced moving their work lives, but also their active lives, into their homes and away from typical common spaces such as gyms or exercise studios. As people began both working and attempting to stay active during quarantine and to practice social distancing, demand for at-home exercise options and equipment has been on the rise. But even at home, could these equipment options pose risks to you and your family? Those injured due to a dangerous piece of exercise equipment may be able to pursue a Texas product liability claim against the manufacturer.
During the course of the COVID-19 global pandemic, tens of millions of people across the country experienced moving their work lives, but also their active lives, into their homes and away from typical common spaces such as gyms or exercise studios. As people began both working and attempting to stay active during quarantine and to practice social distancing, demand for at-home exercise options and equipment has been on the rise. But even at home, could these equipment options pose risks to you and your family? Those injured due to a dangerous piece of exercise equipment may be able to pursue a Texas product liability claim against the manufacturer. In light of COVID-19, everyone seems to be shopping online more frequently. Whether you’re shopping online to adhere to social distancing concerns or simply out of boredom, Amazon has become an important part of regular online shopping trips in many households. When a product purchased from the online retailer, however, injures someone in your family, is Amazon liable in a Texas products liability lawsuit? Or is the entity or individual who sold you the product responsible?
In light of COVID-19, everyone seems to be shopping online more frequently. Whether you’re shopping online to adhere to social distancing concerns or simply out of boredom, Amazon has become an important part of regular online shopping trips in many households. When a product purchased from the online retailer, however, injures someone in your family, is Amazon liable in a Texas products liability lawsuit? Or is the entity or individual who sold you the product responsible? When a consumer purchases a new product, they rightfully trust that the designer, manufacturer, and retailer took measures to ensure the product’s safety and efficacy. However, despite testing standards and federal oversight, some dangerous products make their way into the consumer stream. Products with a design or manufacturing defect or that are inherently dangerous may cause serious injuries and lead to a Texas product liability lawsuit. The United States Consumer Product Safety Commission (USCPSC) requires manufacturers, distributors, and similar entities to report any issues with their products and issue recalls if necessary. However, these parties may still face liability even if they issued a recall.
When a consumer purchases a new product, they rightfully trust that the designer, manufacturer, and retailer took measures to ensure the product’s safety and efficacy. However, despite testing standards and federal oversight, some dangerous products make their way into the consumer stream. Products with a design or manufacturing defect or that are inherently dangerous may cause serious injuries and lead to a Texas product liability lawsuit. The United States Consumer Product Safety Commission (USCPSC) requires manufacturers, distributors, and similar entities to report any issues with their products and issue recalls if necessary. However, these parties may still face liability even if they issued a recall. Following the initial COVID-19 outbreak in the United States earlier this year, thousands of Americans flooded stores in search of hand sanitizer and other cleaning supplies. Many stores were completely wiped out from the start of the pandemic of such supplies and have taken several months to restock these products because of demand. In preparation for the uncertainty associated with the pandemic, many Texans purchased large quantities of these products. However, recent FDA recalls indicate that some of these sanitizing products may be causing members of our community to become ill. These recalls may be the basis for a Texas product liability claim.
Following the initial COVID-19 outbreak in the United States earlier this year, thousands of Americans flooded stores in search of hand sanitizer and other cleaning supplies. Many stores were completely wiped out from the start of the pandemic of such supplies and have taken several months to restock these products because of demand. In preparation for the uncertainty associated with the pandemic, many Texans purchased large quantities of these products. However, recent FDA recalls indicate that some of these sanitizing products may be causing members of our community to become ill. These recalls may be the basis for a Texas product liability claim.